Measure Your Inflammation with the COR One™
FDA-Registered
FSA & HSA Eligible
Medical-Grade
Description
The COR One™ is the first at-home medical device to monitor chronic, acute, and low-grade inflammation with just a single drop of blood.
Inside its compact, lightweight design by Apple Watch architect Bob Messerschmidt is a powerful tool that reveals insights into your body’s unique health patterns.
Benefits
Knowing is half the battle, and COR One™ enables you to see which lifestyle changes really impact reducing inflammation—directly in your home.
Inflammation underpins many chronic conditions, from heart health to metabolism, but generic solutions often miss the mark. COR One™ gives you personalized insights by measuring your unique inflammatory response, showing which actions—diet, supplements, lifestyle, or fitness—reduce inflammation for you.
COR One™ allows you to build a data-driven routine tailored to your body, right from home.
What’s Included
COR One™ Device
Your personal inflammation monitor - free to use during your subscription - connects to Wi-Fi to analyze your results quickly and securely. The device is shipped to you with your first order, keep it as long as you have an active subscription.
COR Mini Test Tubes
Each test tube provides accurate, safe testing at a fraction of traditional lab costs. Test up to 5 times each month.
Sample Station
The Sample Station makes blood collection simple and painless. Compact and portable, it’s easy to use anywhere.
USB-C Cable & Charger
Power up through the included USB-C charger.
Family Mode
Keep multiple profiles on one device. Perfect for managing your whole family's health data separately and securely.
COR One Subscription comes with the COR One device, free to use during your active subscription, as well as all the above accessories, all protected by our lifetime warranty.
If COR is not for you, you can pause or cancel any time.
Shipping
Flat rate shipping in the USA and Canada. We’re committed to making your experience seamless, so your COR One™ device will be carefully packaged and shipped directly to your door. Once your order is placed, you’ll receive a confirmation email with tracking information so you can monitor your delivery every step of the way. Expect your device to arrive within 3-5 business days.
WARRANTY
Your satisfaction and peace of mind are our top priorities. COR One™ comes with a comprehensive warranty to protect against any manufacturing defects. If you experience any issues with your device, simply reach out to our customer support team, and we’ll ensure it’s resolved promptly. For more information on what’s covered under our warranty and how to make a claim, please refer to our warranty policy or contact us directly at hi@corhealth.com.
“I love my device. I've been using it for about 6 months and it's incredibly helpful to see how different foods, supplements, and dietary restrictions affect my personal inflammation levels. The best part is the tests are inexpensive and you test at home and get results in about 30 minutes.”
AS SEEN IN
Inflammation in three simple steps.
The COR One™ takes just 30 minutes to get you your results.

Quick Blood Sample
One tiny drop from a gentle finger prick - as simple as a glucose test.

Let COR One™ Analyze
Your device processes the sample and sends the results straight to your phone.

See What Works
Watch how your diet and lifestyle choices affect your inflammation levels over time, with personalized AI insights to guide you.
Get clear, actionable data about your inflammation.
Get Mine NowTracking with purpose
COR One™ goes beyond lab tests, empowering you to actively improve your health and support a longer life.
Easily record your progress, receive personalized guidance, and understand what truly impacts your inflammation—helping you make meaningful changes over time.
This is a fantastic tool for tracking your inflammation. You can measure it at home instead of going to a clinic and waiting a week for the results. You can measure it weekly, and for that week you do one experiment, such as adding an anti-inflammatory supplement to your daily routine. After a week you can see a result. Did it improve your inflammation or not?
What's Included
Here’s what your complete COR One™ kit includes.

The COR One™ Device
The COR One™ captures and analyzes blood samples through high-quality imaging, connecting to WiFi to securely transmit data to COR’s secure servers for rapid analysis.

Smart Sample Station
The Sample Station makes blood collection simple and painless. Compact and portable, it’s easy to use anywhere.

COR Mini Test Tubes
Each single use test tube provides accurate, safe testing at a fraction of traditional lab costs. Test up to 5 times a month.

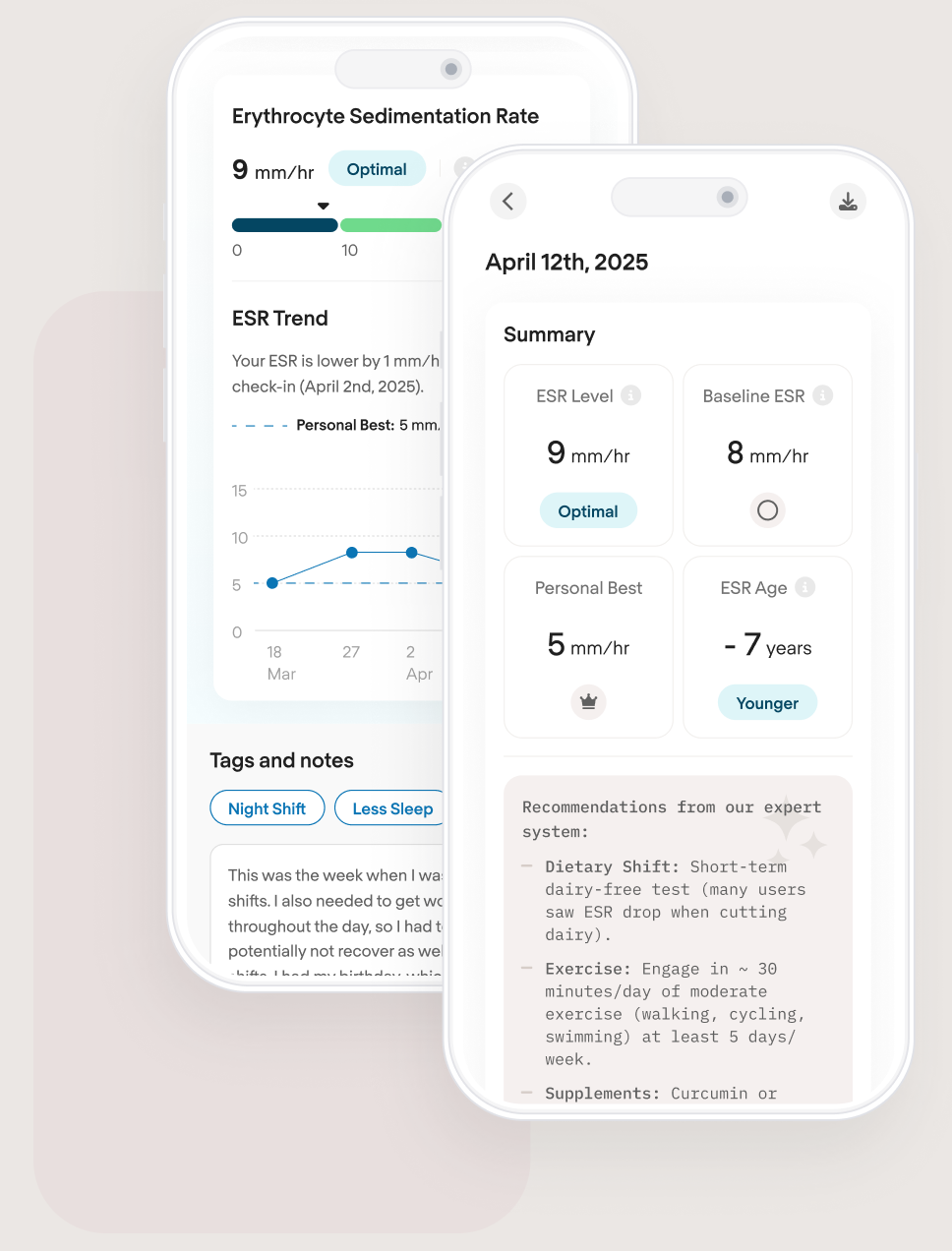
COR Dashboard
Don't settle for average, be optimal. See your progress through the COR dashboard in our web app. No need to download, you get free access. You see your ESR Level, a Baseline ESR which is your best reading each month, your personal best ESR, and your COR ESR Age which tells you how you are doing relative to other COR users your age.
Get clear, actionable data about your inflammation.
Get Mine NowAffordable & Convenient
Get professional-grade results without the lab costs. The COR One™ fits in your hand, plugs into USB, and gives you answers in 30 minutes.

Quick, painless & easy
One tiny drop of blood is all you need – collected with the same gentle finger prick diabetics use for daily testing.

Medically accurate
COR One™ delivers precise inflammation tracking to support accurate health monitoring.

Hear from Thousands Who Have Already Benefited from COR One™
Get clear, actionable data about your inflammation.
Get Mine Now
Frequently
asked questions
Have any questions? We have the answers.
What does this measure?
Sed Rate—short for Erythrocyte Sedimentation Rate (ESR)—is a composite signal of systemic inflammation. It reflects how quickly red blood cells (RBCs) settle to the bottom of a test tube, a process that accelerates in the presence of inflammatory proteins.
Do other home devices exist to measure chronic inflammation?
The COR One™ is the first and only completely at-home solution.
How is Sed Rate different from CRP?
CRP is one inflammatory marker. Sed Rate reflects many inflammatory proteins including CRP—making it better at capturing ongoing or chronic inflammation that CRP alone might miss.
Do you have a CRP tracker as well?
ESR is the best marker for tracking because it captures the full picture — it responds to dozens of different acute-phase proteins, stays stable enough to show real trends, and reflects both acute and low-grade inflammation. CRP can spike and fall in a matter of hours, which makes it easy to miss changes unless you test constantly. That’s why COR One uses ESR — it’s the gold standard for seeing whether your inflammation is truly moving in the right direction.
What is a normal Sed Rate?
It depends on your age and sex, but in general: lower is better. Even within the “normal” range, lower values correlate with better long-term health outcomes. Many of our users aim to be "optimal" with ESR in the 5-7 range or less.
Are COR One™ results as accurate as lab results?
The COR One™ is medically accurate and FDA registered.
Is the COR One™ available internationally?
COR is currently available in the US and Canada. As with all products the COR One™ is subject to local regulation and requirements.
Can I share the results with my physician?
Yes. Your physician will understand your Erythrocyte Sedimentation Rate (ESR) results. Additionally, the COR dashboard allows you to print your results. You can then share them with medical professionals.
Is COR covered by insurance?
According to the Internal Revenue Service, Class 1 medical devices qualify for inclusion in Flexible Spending Accounts and Health Savings Accounts (FSA/HSA) under Internal Revenue Code Section 213(d). The COR One™and COR Mini Test Tubes are coded as health care products and they may be purchased with your FSA or HSA credit card. Please contact support@corhealth.com for a payment link to use HSA/FSA cards.









